
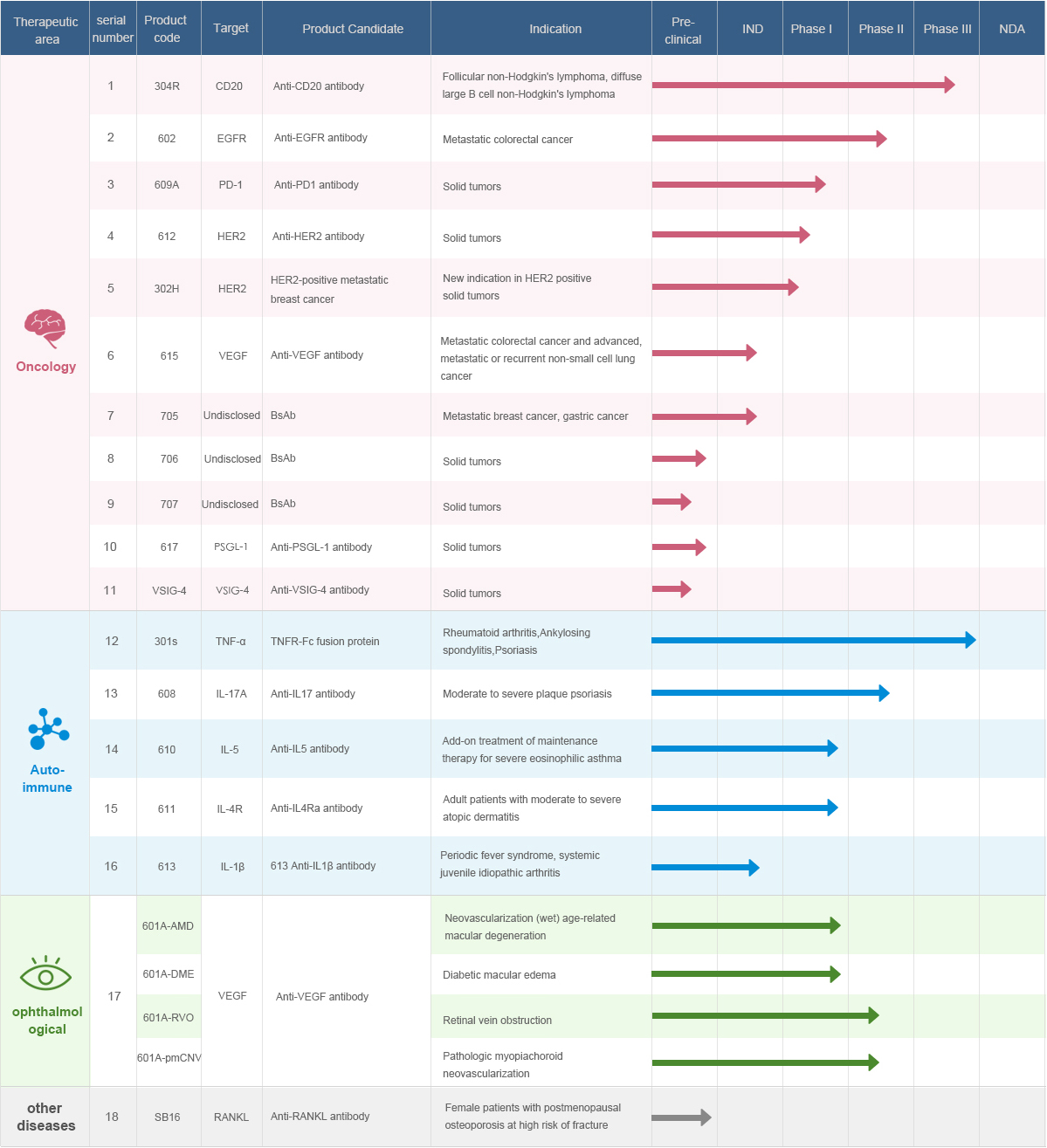
304R (recombinant human-mouse chimeric anti-CD20 monoclonal antibody for injection): The proposed main indication is non-Hodgkin lymphoma and the Phase III clinical trial has been completed.
602 (recombinant human-mouse chimeric anti-EGFR monoclonal antibody for injection): The proposed main indication is metastatic colorectal cancer, and it is currently in the Phase I clinical trial.
601A (recombinant humanized anti-VEGF monoclonal antibody for injection): The proposed main indications are age-related macular degeneration and diabetic macular edema; and it is currently in Phase I clinical trials.
609A (humanized anti-PD-1 monoclonal antibody for injection): The proposed main indication is solid tumors, and the Phase I clinical trials are currently underway in both China and the United States.
301S (recombinant human tumor necrosis factor-α receptor Ⅱ: IgG-Fc fusion protein for injection): The proposed main indications are rheumatoid arthritis, ankylosing spondylitis, and psoriasis.